The AstraZeneca COVID-19 vaccine is a vaccine developed by Oxford University and AstraZeneca. The vaccine was approved for use in the UK on 30th December 2020 and later administered to its first patient in early January 2021. The development, testing, approvals and deployment of such vaccines has been regarded as one of the biggest and most pioneering healthcare feats.
This is a moment to celebrate British innovation – not only are we responsible for discovering the first treatment to reduce mortality for Covid-19, this vaccine will be made available to some of the poorest regions of the world at a low cost, helping protect countless people from this awful disease. It is a tribute to the incredible UK scientists at Oxford University and AstraZeneca whose breakthrough will help to save lives around the world.
Matt Hancock, UK Secretary of State for Health and Social Care

The now infamous conflict that occurred on January 23rd between the EU and AstraZeneca showed a substantial amount of chaos with regards to vaccine supplies.
On 30/01/2021, the EU had released their contract with AstraZeneca. With this redacted contract now public, the EU stated that AstraZeneca has a contractual obligation to provide the vaccine, not an option. The redacted publication details the deal between the two parties for 300 million doses of the vaccine in August 2020. However, this does not settle the issue of which party was at fault here.
EU officials have insisted that their contract with AstraZeneca stipulates the primary manufacturing sites would be based in the UK, rather than in Belgium and the Netherlands. While AstraZeneca representatives state otherwise.
Because of this, the EU has recently been criticised for the slow rollout of the vaccines to its population of 450 million citizens.
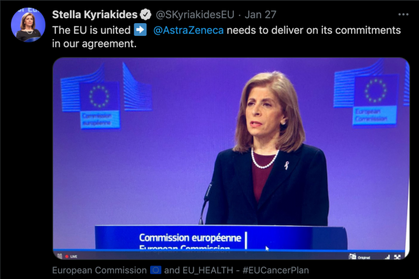
The EU, namely Stella Kyriakidehas, urged the pharmaceutical firm to deliver the vaccine dose as per their contractual duty. A redacted version of this contract was later revealed by the EU, detailing the deal they had made with AstraZeneca. Although a majority of the vaccines are produced in the UK, AstraZeneca states that there are factories based in Belgium and the Netherlands and that this is where the delays are originating;
“Initial volumes will be lower than originally anticipated due to reduced yields at a manufacturing site within our European supply chain”
This has been compared to UK based factories that have had, to date, no problems with manufacturing or the delivery of vaccines, and have been rolling out the vaccine to millions (as of 02/02/2021) for the UK’s mass immunisation programme. The UK is on track to hit its target of vaccinating everyone over the age of 70, as well as those classed as extremely vulnerable. This is a significant milestone compared to other EU states.
AstraZeneca confirmed the shortfall of the doses with European officials, naming manufacturing issues as the problem, but not addressing the magnitude of the shortfall, which is expected to be 60% in the first quarter of 2021.
Reuters reported that the delivery would be reduced to 31 million in the first quarter of this year. Alongside this, the EU also ordered 2.3 billion doses of the coronavirus vaccine from other companies, namely Pfizer/ BioNTech, and Moderna, which they have received.

The pharmaceutical firm states that its existing contract for UK supplies prohibits fulfilling the demands made by the EU. The latter of which called for the diversion of vaccine doses already made by UK factories. AstraZeneca stated that they must fulfil Britain’s order of the vaccine before considering sending the supply abroad
Pascal Soriot, the CEO of AstraZeneca, initially stated that the supply would be in EU states for February and March 2021;
“We will be supplying tens of millions of doses in February and March to the European Union, as we continue to ramp up production volumes,”
Based on this statement, the EU had hoped for delivery of the vaccines to start straight away. With the order of 18 million doses arriving in EU states by March 2021. However, the AstraZeneca CEO had also stated that the production of the vaccine was essentially two months behind where they had originally anticipated.
As a result of this conflict, EU countries were threatening to sue over the delays and subsequently restrict vaccine exports, such as the Pfizer vaccine developed by Germany and the United States, to the UK and other nations.
After discussions on Wednesday between the EU and AstraZeneca, the EU Health Commissioner had expressed regret for AstraZeneca for their “Continued lack of clarity on deliveries” and taken to Twitter to further discuss possible solutions.

With regards to effectiveness, Germany’s vaccine commission has stated that the AstraZeneca vaccine cannot be recommended for use for those aged 65 and over. This is due to the apparent lack of data on how the vaccine affected people of this age group. However, in contradiction to this, several public health officials and other studies have found that the vaccine is safe and provides high levels of protection, cutting the transmission of the virus by two-thirds.
After the European Medicines Agency (EMA) confirmed approval of AstraZeneca’s vaccine, they disclosed that the participants in the study were between the ages of 18 and 35. They later confirmed that there were not enough results to show how this vaccine, compared to Pfizer/BioNTech, and Moderna, had affected those that were 65 and over. Regardless of this, the EMA had approved the use of the vaccine in the EU States, reiterating the 60% effectiveness in clinical trials.
As of 1st February 2021, the EU had stated that the pharmaceutical firm will now supply nine million vaccine doses by March. Ursula von der Leyen described this as ‘a step forward’ however it also depends on AstraZeneca’s likelihood of fulfilling this promise.
OCI’s View
OCI is committed to keeping updated on the latest news and how it affects global and domestic supply chains, especially during this pandemic.
To stay updated please follow OCI on LinkedIn and keep an eye on our website for more news.